-
Structure and properties of complex hydride perovskite materials
P. Schouwink, M.B. Ley, A. Tissot, H. Hagemann, T.R. Jensen, L. Smrcok and R. Cerný
Nature Communications, 5 (2014), p5706


DOI:10.1038/ncomms6706 | unige:43536 | Abstract | Article HTML
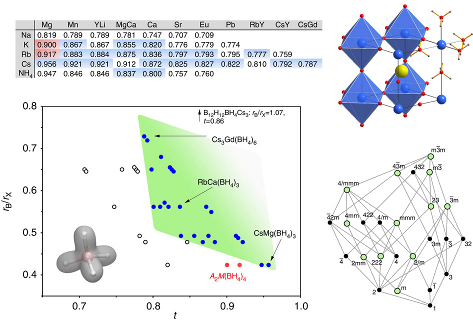
Perovskite materials host an incredible variety of functionalities. Although the lightest element, hydrogen, is rarely encountered in oxide perovskite lattices, it was recently observed as the hydride anion Hâ, substituting for the oxide anion in âBaTiO3. Here we present a series of 30 new complex hydride perovskite-type materials, based on the non-spherical âtetrahydroborate anion âBH4â and new synthesis protocols involving rare-earth elements. Photophysical, electronic and âhydrogen storage properties are discussed, along with counterintuitive trends in structural behaviour. The electronic structure is investigated theoretically with density functional theory solid-state calculations. BH4-specific anion dynamics are introduced to perovskites, mediating mechanisms that freeze lattice instabilities and generate supercells of up to 16 Ã the unit cell volume in AB(BH4)3. In this view, homopolar hydridic di-hydrogen contacts arise as a potential tool with which to tailor crystal symmetries, thus merging concepts of molecular chemistry with ceramic-like host lattices. Furthermore, anion mixing âBH4ââXâ (Xâ=Clâ, Brâ, Iâ) provides a link to the known ABX3 halides.